 ) and brightness (running from black to white, in
the case of colored surfaces, or specified quantitatively on some physical
scale with appropriate units, which we can ignore for the present).
) and brightness (running from black to white, in
the case of colored surfaces, or specified quantitatively on some physical
scale with appropriate units, which we can ignore for the present).
Color is an aspect of vision: strictly speaking, there is no color without a human observer. So we must distinguish between the properties of a visual stimulus that evokes a color sensation, and the sensation it evokes. The former (the physical side of color) is a property of the light entering the eye; the latter (the perceptual side) involves the human visual system, and depends on its state of adaptation, and the visual context of the perceived object.
The physical description of color stimuli is treated in the part of color science known as colorimetry (color measurement). Very extensive color-matching experiments made with many human observers have shown that the retina of a normal eye contains three types of cone-shaped receptors that respond to long-, medium-, and short-wavelength light. The brain processes the signals from these 3 types of cones to produce the sensation of color. However, the color-producing properties of any light are known completely from the strengths of the stimuli presented to the L, M, and S cones; so three numbers suffice to describe a color stimulus.
These three numbers correspond to the fact that perceived
color-space is three-dimensional: all colors can be arranged continuously
in three dimensions.
Unfortunately, people commonly use the word “color” to mean just one of these
dimensions, the one technically called hue
(red,
green,
blue, and
many other “color names” refer to this property of colors.)
The other two dimensions are saturation (how much the color differs
from neutral gray:
 ) and brightness (running from black to white, in
the case of colored surfaces, or specified quantitatively on some physical
scale with appropriate units, which we can ignore for the present).
) and brightness (running from black to white, in
the case of colored surfaces, or specified quantitatively on some physical
scale with appropriate units, which we can ignore for the present).
Though there are other ways of describing the 3 dimensions of color space, I'll just use the ones mentioned above. Hue, saturation, and brightness provide a way to describe colors, without the confusion of terms like “bright” (which sometimes means “high in brightness” but sometimes means “high in saturation”) or “pale” and “light” (high in brightness, or low in saturation?) Obviously, the colors of green flashes are highly saturated.
One way to represent the 3 dimensions of color stimuli is to specify characteristics that correspond roughly to these 3 properties of perceived colors. For example, something that roughly corresponds to hue is the “dominant wavelength” — the place in the spectrum that, roughly speaking, elicits the same hue sensation as the stimulus in question. (Unfortunately, there are “non-spectral” hues, like purple and magenta, that require special treatment in this scheme.) The physical correlate of saturation is called “excitation purity”; it corresponds to the amount of pure spectral light that must be mixed with white light to match the saturation of the stimulus. And the “brightness” of optical stimuli can be measured with instruments that mimic the spectral sensitivity of the eye; for the extended surface colors we are mainly concerned with, the corresponding physical quantity is called “luminance.”
Fortunately, we don't need to get into the details of colorimetry for the present discussion. You just need to be aware that color stimuli and color sensations are apples and pears: they belong to the same family, but are distinctly different members.
It's a common error to suppose that there is a unique correspondence between stimuli and perceptions. However, the color that is perceived depends on other stimuli that have been presented to the same part of the retina, and on those in surrounding areas. Consequently, there is not a unique perceived color for a given stimulus — a fact vividly demonstrated by the 12th demonstration at Dale Purves's website: click on the “Enter” tab at the top left of the main page, then on the “See for yourself” menu item, and use the slider to scroll down to the “Color contrast: Cube” demo. Unfortunately, many physicists are unaware of this fact, and suppose that a given stimulus always evokes the same color sensation — a serious confusion.
A fixed stimulus may be perceived as white, black, yellow, brown, or even green, depending on the eye's state of adaptation, and the context in which the stimulus appears. Purves also has a fine demonstration of a patch that appears either orange or brown, depending on its context; see his demonstration 06, “Brightness contrast with color: cube”.
So it is necessary to distinguish between the color that would be perceived by a “neutrally adapted” eye under standard conditions — which may be called the “nominal color” of the stimulus — and the color actually perceived under some other conditions.
Because the visual system has a remarkable ability to discount the quality of the illumination, a colored surface is perceived to have nearly the same color under a wide range of lighting conditions — a phenomenon called “color constancy.” Color constancy deceives us into thinking that a given surface has a fixed color that is a property of the material itself. (Note that color constancy means that the same perceived color can correspond to a wide variety of physical stimuli. Purves again demonstrates this, in his demonstration 13, “Color constancy: cube”.) Because of this deception, people often ask the meaningless question, “What color is it really?” when presented with optical illusions that depend on color contrast and adaptation.
Color constancy depends on having a wide variety of colored objects in the field of vision. When only a single colored patch is presented — particularly, if it appears against a dark background — the visual system does not have enough cues to “discount the illuminant,” and the stimulus is perceived more nearly as a colored light.
Color constancy involves the relations between the colors of an object and its surroundings; but an isolated patch of color is seen as an “unrelated” object. A common example is the “silvery Moon,” which usually appears white to the eye. Actually, the Moon is about the same color as an old asphalt parking lot: a dark, brownish gray. But, when we see it in a dark sky, it is often the brightest object visible, and we have no white comparison standard to show how dark it really is.
Likewise, the setting Sun is seen more nearly as a light than a surface, and we have nothing else to compare it with. Worse, the brightness of the setting Sun is so great that it usually bleaches the red-sensitive photopigment from the long-wavelength (or “L”) cones, so that the relative signal supplied to the brain by these cones is considerably weaker than it normally would be, by the time a sunset green flash appears. The result is that the flash already appears green at a stage where a normally-adapted eye would see yellow light. (See my paper on this subject for further details.)
Many people suppose that color photographs reproduce colors faithfully, though this is easily shown not to be true (e.g., many common flowers, such as the “Heavenly Blue” morning-glory, are not correctly reproduced by color films.) The properties of available dyes make accurate color reproduction impossible, so the manufacturers of color films have compromised by making their materials reproduce accurately the commonest subjects of their customers: people's faces.
This compromise makes color films reproduce everything else more or less incorrectly. Some colors are reproduced very incorrectly; and unfortunately, this includes the colors of high spectral purity that appear in green flashes. Thus the colors displayed in photographs of green flashes are considerably different from the colors actually seen by human observers.
Bearing in mind these complications, let's now consider just the physical side of sunset colors. The sunlight travels through the atmosphere, which reddens it by both molecular scattering (Rayleigh scattering) and aerosol scattering. (Both these processes remove more of the short-wavelength light, with nominal colors like blue and violet, than of the longer wavelengths, like red; the selective extinction of the shorter wavelengths leaves the average color of the remaining transmitted light much redder.) There is also some absorption, primarily by the ozone bands in the part of the spectrum that ordinarily appears orange.
The amount of molecular scattering and absorption depends on the amount of air in the line of sight. At the horizon, there is about 40 times as much air between you and the Sun as there would be if the Sun were directly overhead (a condition not observable outside the Tropics, I should point out.)
However, the aerosol is concentrated toward the bottom of the atmosphere. Because this aerosol layer is relatively thin, there can be 100 or 200 times more aerosol in the line of sight at the horizon than there is directly overhead. So, even though the aerosol isn't inherently as strongly reddening as pure air, the relatively large amount of aerosol at the horizon can produce more reddening than does the gaseous part of the atmosphere.
Because both the total amount of aerosol and its vertical distribution change a great deal from day to day, the color of the Sun and sky near the horizon also change a great deal. If the air is very clear, the low Sun can be very bright, and of a nominally orange color (though it often looks yellow rather than orange). If the aerosol layer is shallow and dense, the low Sun is deep red.
The result of the atmospheric reddening is that the brightness of the low Sun falls off very rapidly at the shorter wavelengths; the more aerosol in the line of sight, the faster the short wavelengths die out. If there is a great deal of aerosol concentrated in a thin layer near the Earth's surface, the Sun can disappear completely before it reaches the horizon.
Now consider what happens at sunset when part of the solar image is cut off geometrically, either by the horizon, or by the optics of a mirage. Atmospheric refraction raises the Sun's image above its geometric position; because of the dispersion of air, this effect is greater at shorter wavelengths. So the long-wavelength images of the Sun “set” (or disappear from mirages) before the short-wavelength images, which disappear later: the geometric cutoff removes the longest wavelengths first, briefly leaving the shorter ones visible. But, because of the rapid falloff of brightness with decreasing wavelength, the light remaining at every stage is mostly just on the short-wavelength side of the geometric cutoff.
When the red and orange are gone, what remains is mostly yellow. When the yellow is gone, what's left is mostly green; when the green goes, the rest is mostly blue. So the nominal color of the remaining piece of Sun rapidly runs toward the short end of the spectrum, as the observed colors of green flashes show. (The simulated examples also show how the color depends on the cutoff wavelength.)
Of course, at some point, what's left is too dim to see against the sky; then the Sun disappears. People usually seem to remember the last color they've seen, so if that was green, the flash is called “green”; if the last color was blue, it's a “blue flash,” and so on. Actually, if you pay close attention, you can see the color running through the spectral colors in turn, until the remaining spot is too faint to see against the sky. Watch for this in the simulations.
A curious result of the rapid increase in aerosol extinction is that more aerosol makes the color of the flash purer. At first glance, this seems paradoxical: the aerosol is reddening; so how can it make a green flash greener?
The answer is that it makes a green flash greener by more effectively eliminating the blue that would dilute the green if the air were clearer. Atmospheric refraction cuts off the long wavelengths; aerosols (and molecular scattering) cut off the short ones. The more effective this aerosol cutoff is, the purer the color of the flash — even though the flash is also dimmer.
But that's only true as long as the flash is appreciably brighter than the surrounding sky. More aerosol extinction makes the flash fainter, as well as higher in purity. And more aerosol scattering makes the sky brighter. So clear-sky flashes, while less pure in color than those seen in hazier conditions, are more likely to persist to the blue, or even the violet, stage. If the air is very hazy, even the green stage may not be reached before the flash disappears into the bright sky. (See the examples that show how aerosol scattering affects the final colors of flashes.)
So dim, red sunsets are unfavorable for green flashes. On the other hand, if the air is very clear, part of the Sun may remain visible in blue or even violet light after the green image has disappeared; then we have a blue or a violet flash. And, as there's less extinction at greater heights above sea level, the chances of seeing a blue or violet flash are increased by going to the mountains; see Robert Wagner's pictures taken from a height of 2200 meters, or the pictures taken from Pic du Midi (over 2800 m) and from a mountain in Chile (on the pictures page) for good examples.
But the human observer usually does not see these nominal colors. If you have been watching the setting Sun, you've exposed your retina to very bright red light. That bleaches some of the red-sensitive photopigment in the L cones. The less-sensitive cones now tell your brain there's less red light, so instead of looking orange, the setting Sun looks yellow; or, instead of red (if there's a lot of aerosol), it looks orange.
The time scale for these changes in sensitivity is typically several seconds, or even a minute. You're unaware that your color vision has (temporarily) changed. But, when the flash arrives, the stage that is nominally yellow — and that appears yellow to someone who has not been watching the sunset, and just looks at the last moment — looks green to you. (Remember that “yellow” is a color seen when the L and M cones are roughly equally stimulated.)
If you're standing there with a camera, you trip the stutter as soon as you see “green,” and get a yellow (or even an orange) flash reproduced on the film, and your friends scoff, and tell you there's no such thing as a green flash.
But if you had waited half a second or so, the nominally (and photographically) yellow stage would have passed on to nominally green. If the air was clear enough for this nominally green stage to be seen, your friends would have been impressed and you'd have been “the hero of the day” (as one famous GF observer once was, on showing his friends a nice sunset display.)
Of course, at sunrise, you don't have these problems with color perception. The first gleam of the rising Sun can be emerald green, or (in the mountains, where the air is clearer) even brilliant blue. You have to know where to look — especially because, in very clear air, there's no aureole of scattered light to announce the spot where the Sun will rise. But the colors seen will be closer to the nominal ones.
That's assuming you have normal color vision, of course. I often wondered whether a “color-blind” person could see any trace of a green flash. Finally, I have the answer: no. My ophthalmologist, who lives near the beach, and enjoys green flashes, has had to put up for years with three friends who claimed green flashes don't exist. Finally, he took his book of Ishihara plates home and tested their color vision: they all turned out to be deuteranopes.
Unfortunately, even at sunrise, it's difficult to photograph green flashes and have the color on the photograph close to what you saw. The reason is that color films produce very poor renditions of spectrally pure colors, and flashes have high spectral purity.
The photosensitive pigment in the eye have action spectra that overlap
considerably. So if you scan through the spectrum, you see a smooth,
continuous change of color from short to long wavelengths, something like
this:
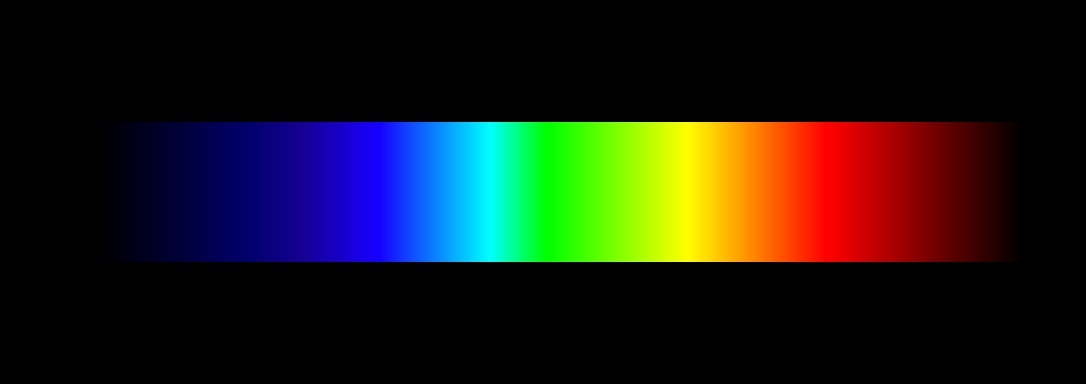
But if you try to photograph the spectrum, you get very strange-looking results. If the image is underexposed, it consists of three uniform blobs of color: red, green, and the thing that the photographic industry calls “blue”, but is really a blue-violet color. As the exposure increases, a little piece of yellow appears where the red and green overlap, and a piece of sky-blue (which the photographers call “cyan”) appears where the “blue” overlaps with the green. Hardly any intermediate colors appear at all (for example, there's very little that appears orange in hue.) When the spectrum is overexposed, the yellow and cyan patches spread into their neighbors more and more.
The result is that actual green flashes, which show an appreciable gradation of hue, often turn out quite uniform in hue — and frequently the hue on the photograph is quite different from that seen by the eye. On top of this, even the best color films can't reproduce colors of the high spectral purity that green flashes have; so the colors on photographs seem dull and lifeless by comparison. Likewise, the colors that can be produced on a computer monitor are less saturated than those of green flashes.
Nevertheless, with practice and luck, some quite nice photographs can be obtained, as our “picture page” and the selected examples of observed colors of flashes show. So it's worth trying to photograph green flashes, even if it's just to prove to your friends that you really did see one!
At this point, you may be ready to have a look at the general advice on how to observe green flashes, or how to photograph them. If so, good hunting!
© 2001 – 2003, 2005, 2006, 2011, 2012 Andrew T. Young
Back to the GF home page
Back to the alphabetic index
Back to the site overview