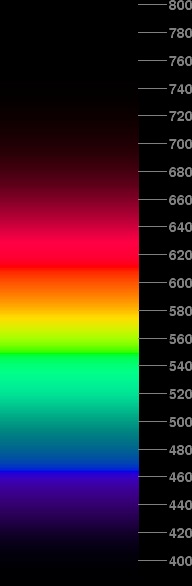
The Latin name “spectrum” (plural: spectra) was given by Isaac Newton to the band of colors produced from white light by dispersing it with a glass prism. These days, we continue to use this name in referring to this sequence of colors, which is seen when light is sorted into its component parts in order of their wavelength, even if the light wasn't white to begin with (so that many, or even most, of the components are missing), and even if the sorting is accomplished by some means other than prismatic dispersion.

Newton rather arbitrarily assigned seven color names, which today we would call “hues”, to parts of the spectrum: red, orange, yellow, green, blue, indigo, and violet. But you can see that the color varies continuously from one end to the other; there is no natural demarcation between one hue and the next.
Some people these days are confused by Newton's use of the terms “blue” and “indigo”. They have forgotten that indigo is the dark purplish-blue dye used to color denim; a new pair of “blue jeans” is exactly the color Newton had in mind when he said “indigo”. That means that his “blue” is a greener hue, more like that of the blue sky. It's approximately the hue called “cyan” today. (When I was a schoolboy, this lighter blue sometimes was called “aquamarine” blue, and indigo was called “ultramarine”.)
The scale at the right side of the simulated spectrum shown here gives the wavelengths of the light in different parts of the spectrum. The unit used is nanometers (1 nm = 10-9 meter, or a millionth of a millimeter). You can see that Newton's “blue” light has a wave length of about 475 nm, while “indigo” is about 460 or 465 nm. Violet light is the stuff at the extreme short-wavelength end of the spectrum.
It's good to remember that the color sensation elicited by a particular light is not (as Newton seems to have supposed) unique and unchanging, but depends on the state of the observer's eye. The standard association of color (hue) names with wavelength intervals in the spectrum should be regarded as a nominal convention; nominally yellow light can appear green to the eye after exposure to bright red light, and this complicates the interpretation of colors seen visually in green flashes at sunset. (I have a paper here that discusses this problem.)
Many people are accustomed to calling the array of colors depicted here a “rainbow”, but that's an error. Rainbows are indeed spectra (of very low quality, as their colors are very dilute and impure); but not all spectra are rainbows. Rainbows are produced by the dispersion of sunlight by refraction in rain drops.
If you want to know more about rainbows, I highly recommend the book “The Rainbow Bridge” by Ray Lee and Alistair Fraser, which is full of beautiful photographs of rainbows, both in Nature and in art. Robert Greenler's book “Rainbows, Halos and Glories” is also excellent. Then there's Minnaert's classic book. On the Web, Prof. Fu-Kwun Hwang has a very nice Java applet that shows all the rays that make both primary and secondary rainbows, and lets you manipulate them. And Les Cowley has added a fine rainbow page to his site, which includes photographs and accurate simulations of rarely-noticed features like supernumerary bows, 3rd-order bows, and twinned bows due to non-spherical raindrops.
But there are other phenomena of atmospheric optics that display the colors of the rainbow, such as the common 22-degree halo, and especially other halo phenomena associated with the “large” (46-degree) halo, such as the brilliantly colored circum-zenithal arc; halos are produced by dispersion in ice crystals. See Walter Tape's book:
W. Tape
Atmospheric Halos, Antarctic Research Series, Vol. 64
(American Geophysical Union, 1994)
for full details. Halos are also well covered in Greenler's book “Rainbows, Halos and Glories”, and in Lynch and Livingston's Color and Light in Nature, 2nd ed. (Cambridge Univ. Press, Cambridge, 2001)
The spectra mentioned so far are produced by refraction (or, strictly speaking, by its dispersion): the refractive index of the refracting material — glass, water, or air — varies with the wavelength of the light, so the deviation of the refracted rays is different for different colors of light (though not by very much). But this produces spectra that are compressed at the red end and drawn out at the violet end, compared to the picture shown above, which has a linear wavelength scale.
To get a spectrum linear in wavelength, you need to use a different process to disperse the light: diffraction and interference. Although we usually think of diffraction gratings as expensive laboratory equipment, they were inspired by everyday materials — such as fine silk cloth, and birds' feathers — that have regularly spaced fine structure. Look at a distant street light through a feather, or fine cloth, and you'll see spectra of its light that are linear in wavelength.
Other artifacts that have the regular fine structure needed to produce spectra by diffraction are phonograph records and CD-ROMs. And small “replica” gratings are commonly used in jewelry today, as well — not to mention “rainbow holograms” that have become common security features of credit cards.
You can often use these everyday spectra to see the lines of mercury vapor in the light of a fluorescent lamp, or to detect the monochromatic nature of light from low-pressure sodium-vapor street lights. The modern world is full of spectra, if you know where to look for them.
© 2002 – 2006, 2011 – 2013, 2025 Andrew T. Young